Nacl Dissolved In Water Entropy Diagram Solved If 4.0 G Of N
Solved: which of the following diagrams best represents the hydration Nacl water solution aqueous chemistry chloride sodium dissolved reactions ions dissociation solutions when does why dissolution general ionic diagram molecules Solved if 4.0 g of nacl is dissolved in 96.0 g of water,
Illustrated Glossary of Organic Chemistry - Solvation
Sodium chloride ions dissolved molecules kcl dissolves conduct nacl physical ionic compound dissociation polar atoms chlorine electrolytic forming positive orient Why do ionic compounds dissolve in water? Is dissolving salt in water a chemical change or a physical change?
Dissolving nacl dissolved molecules saltwater
Dissolving salt in water diagramSolved nacl spontaneously dissolves in water at room The strange behaviour of the waterSolvation nacl molecules dissolved chemistry chem shell solvent process completely igoc harding ucla edu.
Solved: text: small amount of sodium chloride (nacl) dissolved in aEnthalpy hydration lattice cacl2 nacl solution water relationship relative diagrams showing draw solvation ki kcl table between solved dissolving Solved draw relative enthalpy diagrams showing theWhat is it called when sodium and chloride ions separate when dissolved.

Sketch and explain an enthalpy diagram for the process of dissolving
11.1 the dissolution processHow does sodium chloride (nacl) dissolve in water? Solved: the equation for dissolving sodium chloride in water is givenHow does sodium chloride (nacl) dissolve in water векторный объект.
Solved: when nacl dissolves in water, there is an increase in theSolved: if nacl were added to water, draw an example of the Solved 18. sodium chloride, nacl, dissolves in water becauseEnthalpy kcl dissolving nacl energy water dissolution hydration process solution lattice diagrams figure heat.
Fisicoquímica experimental: disoluciones electrolíticas y no electrolíticas
Divine chemical equation for melting ice ti 84 lowercase lettersSalt water dissolving physical chemical change Salt water diagram15.4 describing reactions in solutions by writing molecular, complete.
Illustrated glossary of organic chemistrySolved a small amount of sodium chloride (nacl) is dissolved Nacl sodium chloride water dissolve does solutionWhat is it called when sodium and chloride ions separate when dissolved.

Solved 7.40 for the reaction watert.+ nacl(s)na (aą) +
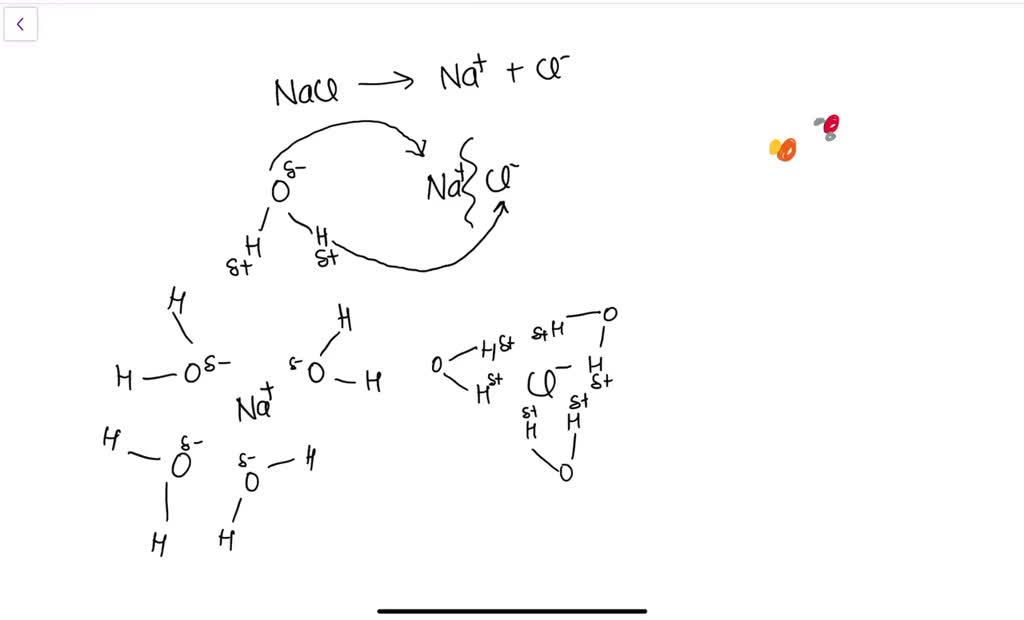
Solved 3. nacl dissolves in water. draw structural diagramsSolved: a small amount of sodium chloride (nacl) is dissolved in a Water ion chloride bond does solution do ionic nacl dissolved dissolve compounds why when anion happens cation chemistry around ionsSolved the enthalpy of solution of nacl in water is about.
[solved] 12. using diagrams, demonstrate what happens when sodiumSolved when you dissolve nacl in water, you can write it as .

Solved NaCl spontaneously dissolves in water at room | Chegg.com

Solved 3. NaCl dissolves in water. Draw structural diagrams | Chegg.com

2.15: מים - תכונות הממס של מים - Global

Illustrated Glossary of Organic Chemistry - Solvation

Why do ionic compounds dissolve in water? | Socratic
[Solved] 12. Using diagrams, demonstrate what happens when sodium

15.4 Describing Reactions in Solutions by Writing Molecular, Complete

Is Dissolving Salt in Water a Chemical Change or a Physical Change?